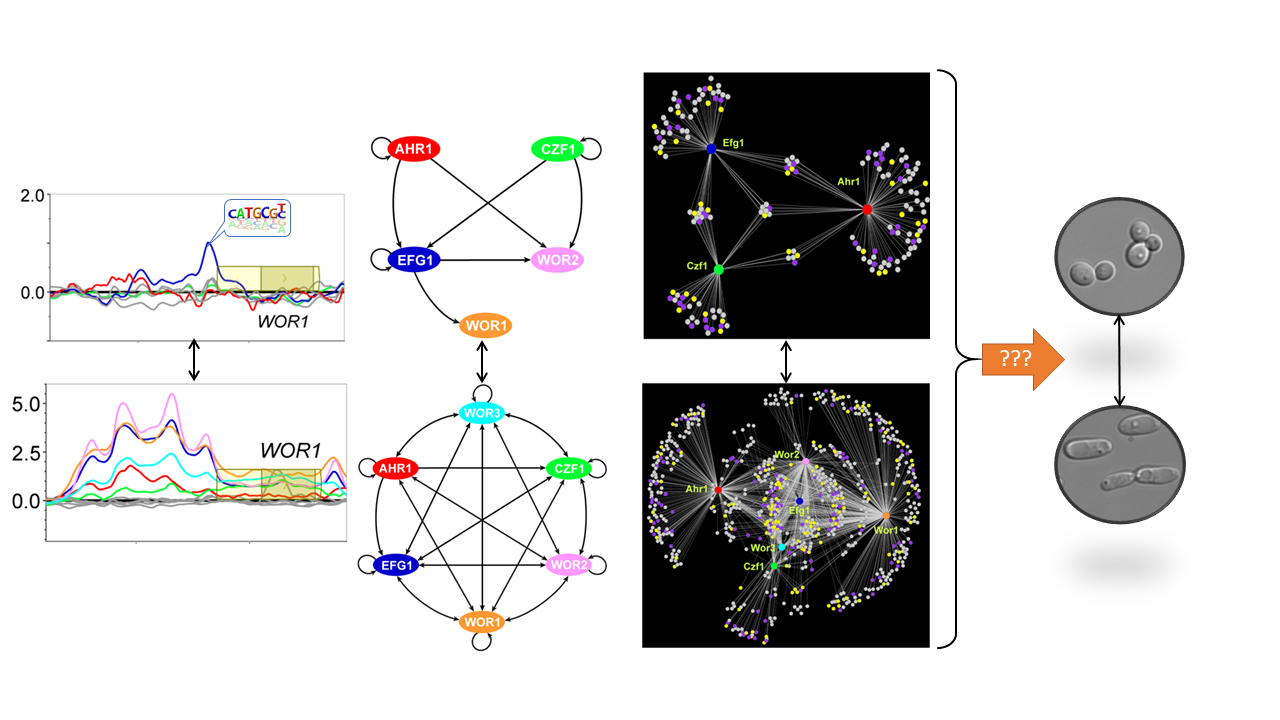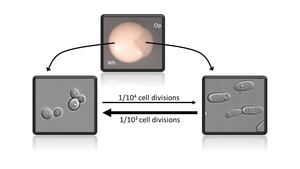
Cell fate decisions, and the subsequent maintenance of unique cellular identities, represent essential processes in the development of multicellular organisms. Similar cell fate decisions play critical roles in the adaptation of unicellular microbes to distinct environmental niches, including during commensal and pathogenic growth within mammalian hosts. The white-opaque phenotypic switch in Candida albicans represents a unique model system for understanding epigenetic heritability in a relatively simple eukaryotic microbe. This switch controls stochastic differentiation between two distinct cell types, each of which is heritably maintained for hundreds of generations. Essentially this switch generates two distinct pathogenic cell types from the same genome. The Hernday lab is focused on utilizing this switch as a model system for developing a detailed, comprehensive, predictive model of the logic and dynamics of a heritable yet reversible transcriptional program in eukaryotes. This model system presents many unique advantages relative to higher eukaryotes, including a small genome size, facile molecular genetics and biochemistry, and the prior identification of all known or predicted transcription factors that are involved in regulating this switch; a similar switch is not found in traditional model yeasts. In addition to gaining insight into the inner workings of complex heritable transcriptional programs, we also aim to develop stable yet reversible engineered transcriptional programs for applied synthetic biology.