Membrane transport proteins constitute an important part of our genome and are target to many therapeutic drugs, but the complexities inherent to their hydrophobicity have significantly delayed their biochemical and structural studies. ATP binding cassette (ABC) transporters is a large family of proteins that use energy from ATP hydrolysis to move a diverse variety of molecules against their concentration gradient across the membrane. ABC transporters are involved in multiple processes: protection against xenobiotics, problems related to drug/drug interactions, development of multidrug resistance, etc., and their malfunctioning can cause terrible diseases such as Cystic Fibrosis or Stargardt. Despite the importance of these proteins, the molecular details of substrate/drugs recognition and the conformational changes that take place during their transport cycle are still unknown. Our lab wants to contribute to a better understanding of these proteins, and for that we do biochemical, spectroscopic, and structural studies of diverse ABC transporters.
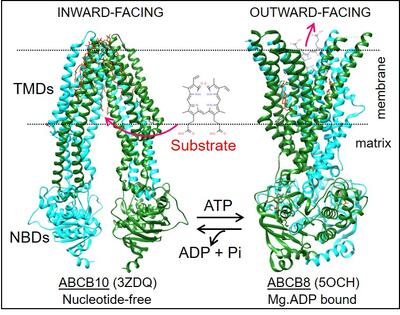
Currently our research is mainly focused on the little studied human mitochondrial ABC transporters. The human mitochondrial inner membrane has three ABC transporters: ABCB7 implicated in iron transport (with mutations causing X-linked sideroblastic anemia with ataxia), ABCB8 involved in regulation of a mitochondrial K+ channel and protection against doxorubicin toxicity, and ABCB10, which is essential for red blood cell development, protection against oxidative stress and whose complete absence leads to death of mice embryos. Some of our goals are 1) developing approaches for the fast and inexpensive production of these human transporters in bacteria for their biochemical and structural analysis, 2) Identifying potential physiological substrates and regulatory molecules that could have therapeutical implications, 3) Determining the molecular basis of substrates-transporter interactions that are essential for their functioning, 4) Studying the effect of disease-causing mutations, and 5) Studying the dynamic conformational changes underlying their functioning, while the transporter is in "near-native" conditions (embeded in a lipid membrane and at physiological temperature).
NEWS: https://news.ucmerced.edu/news/2022/lab-aims-understand-transporter-protein-protects-cells-damage
