Research
The Oviedo Lab aims to understand mechanisms of stem cell regulation during tissue renewal, regeneration, and tumor formation. We use planarian flatworms as model organisms because of their stem cell-based capacity to renew and repair tissues. Planarians can regenerate entire body parts after injury by activating signaling pathways that instruct stem cells. Extensive experimental evidence indicates that the mechanisms regulating stem cell behavior are evolutionarily conserved across the animal kingdom. Therefore, our studies in a simplified innovative model system are expected to reveal critical molecular players, enable novel therapeutic approaches to combat infections and cancer, and promote selective tissue replacement. Continue reading below to learn more about the main research areas in the Oviedo Lab at UC Merced.
Stem Cell Response to DNA Damage and Repair
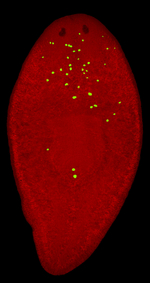
The DNA within planarian cells experiences damage as its human counterpart, and we discovered that the strategies to repair DNA in flatworms are evolutionarily conserved (Barghouth et al., 2020; Barghouth et al., 2019). We developed a new model that facilitates studies of DNA damage and the mechanisms regulating its repair (Peiris et al., 2016). This is significant because DNA damage tends to be highly toxic (i.e., lethal), which generally precludes studies on the complexity of the whole organism. Our model not only overcomes limitations in the field but also allows for using reagents originated in humans (e.g., antibodies). We demonstrated that genetic disturbance of Rad51 -an essential protein for DNA repair, leads to massive amounts of DNA damage throughout the body. Intriguingly, some cells with DNA damage bypass endogenous surveillance mechanisms to survive and divide (yellow dots in picture), while others die, as expected. This important finding is now used to investigate how cells with DNA damage escape cell death and continue to proliferate, a pervasive cancer hallmark. Recently, we identified a group of neurons in the planarian brain that activates the Hedgehog signaling pathway to induce cell death in the presence of genomic instability (Thiruvalluvan et al., 2018). This exciting finding suggests that the fate of cells with DNA damage is modulated by long-range communication originating in the brain. |
Bioelectric Regulation of Stem Cell Behavior
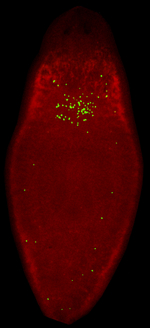
The stimulation of tissues with direct electric stimulation (DCS) is an effective strategy to enhance bone repair, treat neurological ailments such as depression, migraine, Parkinson, and Alzheimer’s disease, and improve mental skills, including multitasking capability and numerical cognition in healthy humans (Aleem et al., 2016; Hao et al., 2015; Kadosh et al., 2010; Nelson et al., 2016). The cellular responses to DCS are consistently observed across invertebrate and vertebrate organisms, implicating robust evolutionarily conserved mechanisms. Despite the extensive use of DCS in experimental, clinical, and public settings, the molecular bases of its effects remain poorly understood. We developed a strategy based on the application of physiological strength DCS that impacts the behavior of different cell types in planarians. We have developed protocols using the most common forms of electric stimulation used in humans: steady-state and pulsing DCS. Our group introduced novel methodologies and equipment to trace, in real-time, DCS-induced molecular responses. We coined this planaria-based approach, “pDCS.” We discovered that it only requires several minutes of pDCS to alter transcriptional activity and cell cycle dynamics and enhance DNA repair without genetic or pharmacological treatments (Davidian et al., 2021, 2022). The image above shows a pDCS-treated planarian displaying stem cell division (green dots) in ectopic areas where neoblasts have been eliminated by X-ray treatment. |
Host-Pathogen Interaction During Fungal Infections
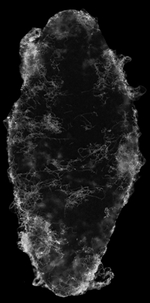
Fungal infections rank among the top ten causes of human death worldwide (WHO, 2018). Candida albicans, for example, is one of the most common fungal pathogens of humans that causes superficial cutaneous and mucosal infections as well as systemic infections resulting in over 40% mortality (Bongomin et al., 2017; Brown et al., 2012). We joined forces with the Nobile lab at UC Merced (expert in fungal pathogenesis) and discovered that planaria flatworms rapidly eliminate infections of pathogenic fungi by activating innate immune responses (Maciel et al., 2019). We developed a fungal infection strategy using Candida albicans as the pathogen and the planaria Schmidtea mediterranea as the host (planarian with C. albicans infection -white signal in picture). Our research team established protocols to visualize C. albicans distribution during the infection of planarian tissues. These analyses determined that fungi rapidly infect and grow in planaria causing damage to superficial and deep tissues. Despite this aggressive pathogen invasion, it only takes a few days for the host to halt C. albicans growth and clear the infection while reestablishing the form and function of damaged tissues. This research introduced planarians as a tractable model organism to study fungal infections.
|
Stem Cell Regulation During Regeneration and Cancer
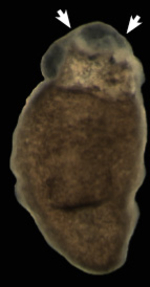
We have characterized metabolic regulators of stem cell responses in planarians. In particular, we have focused on two protein kinases, AKT (also known as Protein Kinase B) and TOR (target of rapamycin), a signaling axis that integrates local and systemic information central to cellular and organismal physiology (Peiris et al., 2016; Peiris et al., 2012). We discovered that regeneration without TOR signaling proceeds through tissue remodeling involving a limited stem cell pool that cannot contribute to animal growth or regeneration. We also identified that AKT signaling plays a critical role in early events of tissue injury. Recently, we published novel roles for Sirtuin signaling as a regulator of animal growth (Ziman et al., 2020). This is interesting because Sirtuins are also present in humans, and drugs used to treat patients produce similar effects in planarians. Malignant transformation is the process by which cells acquire cancer properties. Despite its central role in cancer, how cellular transformation is established remains poorly understood. We developed the first planarian model to study cancer based on the functional disruption of PTEN. This tumor suppressor is one of the most commonly inactivated genes in human cancers (Oviedo et al., 2008). Our recent results suggest that neoblast transition to malignancy can be traced in situ as they attend to demands of tissue renewal. We have characterized molecular events of cell transformation and identified genetic alteration and cellular changes in stem cells as cancer initiates. We have implicated a crosstalk between neural signals and stem cells that facilitates survival and uncontrolled proliferation of abnormal cells (Barghouth et al., 2023). The image shows an advanced cancer-like phenotype in planarians (tumors in the anterior part of the animal, arrows). |

