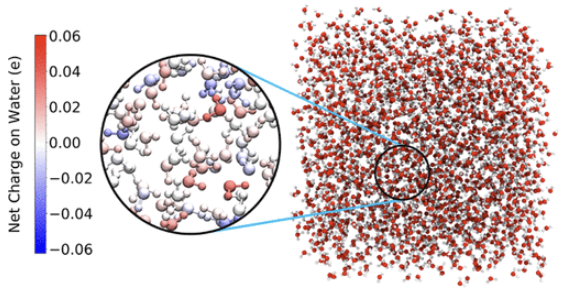
In this collaborative paper published in JCTC with the Shi group, we systematically assess various electronic structure methods and charge models for assigning partial atomic charges to isolated water, water clusters, and liquid water by comparing their predicted dipole moments against available experimental data and examining their capabilities of generating physically meaningful trends. The performances of quantum-mechanical models in assigning partial atomic charge have been discussed before for isolated water and water dimer, but no systematic study is reported on liquid water to the best of our knowledge, and the goal of this work is to address this issue. Although we find that none of the charge models considered in this work (i.e., Mulliken, NPA, CHelpG, RESP, Hirshfeld, Iterative Hirshfeld, and Bader) is capable of fully capturing the observed dipole moment increase from isolated water (1.85 D) to liquid water (2.9 D), we identify a computationally affordable pragmatic charge assigning protocol for liquid water, namely the Iterative Hirshfeld charge model with M06-HF/aug-cc-pVDZ and an explicit quantum treatment of the first two solvation shells of the water molecule of interest, which reproduces the experimental molecular dipole and generates sensible charge transfer for liquid water. We expect that this work will be of broad interest to the quantum chemists developing charge models, and the simulation communities developing and using water models.
