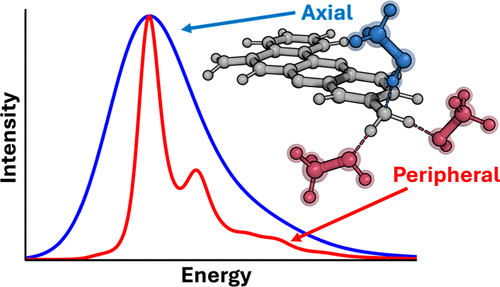
This manuscript examines the effect of specific solvent interactions on the simulated spectral densities, reorganization energies, and absorption lineshapes of the cresyl violet chromophore in methanol. Employing a molecular dynamics trajectory-based approach (both force field and ab initio MD), our study reveals that polarization of the chromophore by solvent in the axial region is responsible for the majority of the inhomogeneous broadening, with one single axial hydrogen bond dominating the broadening. We also show that the strong hydrogen bonds peripheral to the chromophore do not contribute to inhomogeneous broadening but play a key role in controlling the vibronic coupling, and therefore, careful treatment is essential to capture the correct vibronic broadening. The results provide physical insight into the role of specific solvent interactions on the resulting optical spectroscopy.
