 In this manuscript, we examine the role of chromophore-ion interactions on the computed absorption spectrum of the aqueously solvated chromophore anion of green fluorescent protein. Most simulated solution-phase absorption spectra ignore the effects of a heterogeneous environment imparted by an electrolyte solution, often resorting to a continuum solvent approach or the infinitely dilute limit with explicit solvent. However, many experiments take place in complex environments, such as those with high ionic strength. Here we simulate the spectrum of a chromophore in a 1M aqueous NaCl, disentangling the how specific chromophore-ion interactions affect the excitation energies and absorption spectrum. In particular, we focused on discovering if the missing ionic environment in previous simulations would lead to additional spectral broadening, leading to better agreement between experimental and computed spectra.
In this manuscript, we examine the role of chromophore-ion interactions on the computed absorption spectrum of the aqueously solvated chromophore anion of green fluorescent protein. Most simulated solution-phase absorption spectra ignore the effects of a heterogeneous environment imparted by an electrolyte solution, often resorting to a continuum solvent approach or the infinitely dilute limit with explicit solvent. However, many experiments take place in complex environments, such as those with high ionic strength. Here we simulate the spectrum of a chromophore in a 1M aqueous NaCl, disentangling the how specific chromophore-ion interactions affect the excitation energies and absorption spectrum. In particular, we focused on discovering if the missing ionic environment in previous simulations would lead to additional spectral broadening, leading to better agreement between experimental and computed spectra.
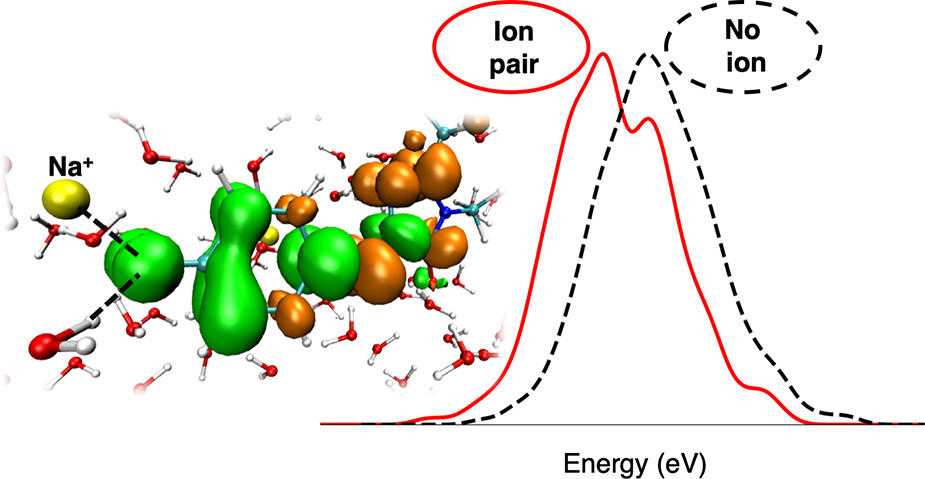
We find that aqueous solvent, treated with either quantum mechanics or molecular mechanics, effectively screens the chromophore from the ionic environment beyond the first solvation shell. Contact ion pairs between the chromophore and Na+ lead to spectral shifts to higher and lower energies depending on the oxygen site of the ion pair formation. Upon investigating these energy shifts, we find that they are surprisingly not due to electrostatic presence of the ion, but instead are due to the ion weakening the hydrogen bond network at the ion pair site. Treating the environment quantum mechanically is key to capturing these spectral shifts. This work provides new physical insight into how chromophore-ion interactions affect the excitation energy and optical spectroscopy of aqueously solvated systems.
