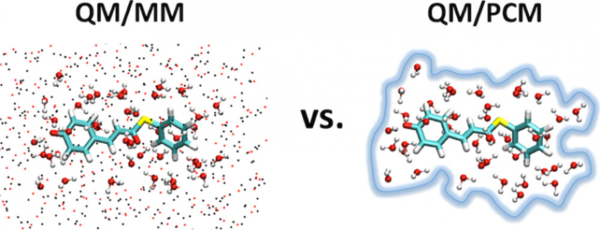
Makenzie's paper (with undergrad Thomas Peev and high school student Chou Xiong) is accepted in J. Phys. Chem. B. This paper describes how to mix explicit quantum solvent with classical continuum and point charge models to obtain converged excitation energies in solution. We compared the effects of a polarizable continuum model (PCM) and molecular mechanical (MM) point charges on the convergence of electronic excitation energies of six solutes in solution using a combined quantum mechanical and classical (QM/classical) approach. With the advent of more efficient computational hardware and software, many studies are now mixing explicit QM solvent with classical solvent models to improve the accuracy of solute/solvent interactions. However, the amount of explicit solvent necessary to reach converged values has not been investigated extensively, and we can find no in-depth comparisons of QM/MM and QM/PCM solvation models. It is thus an open question whether or not the specific MM point charges provide better long-range solvation behavior or whether the polarizable continuum performs better; ideally those studying condensed phase reactivity would like to know which method can be used with fewer explicit QM solvent molecules. Here, we systematically increased the amount of explicit QM solvent to determine the convergence of QM/classical excitation energies with respect to QM region size and, for the first time, directly compare the effects of using PCM verses MM point charges as the classical solvent model. We showed that a relatively large amount of explicit QM solvent (about one solvation shell) is necessary to converge QM/classical excitation energies in aqueous solution. Surprisingly, QM/PCM does not offer faster convergence despite its inclusion of mutual polarization between the QM and classical regions.