Developing self-assembly methods to arrange individual molecules and atoms precisely in space and time represents a powerful approach for creating new materials that can unlock future technology. This emerging concept of "growing materials" from the bottom-up provides the intellectual underpinning that motivates research within the Merg lab. Specifically, we are interested in the design, synthesis, and assembly of unexplored peptide building block designs for creating novel peptide-based architectures with tailorable physical and chemical properties - an outstanding challenge within the field of molecular self-assembly.
A common thread within the lab centers around employing peptides as fully synthetic, highly modular biomolecular building blocks. Peptides represent an attractive biomacromolecular building block because of the ability to encode a large amount of structural, chemical, and functional information within the sequence architecture (as evidenced by the exquisite sequence-to-structure-to-function relationship exhibited by proteins). Furthermore, the high-information content of peptides and their conjugates can be programmed and postsynthetically modified using a range of chemical synthesis techniques, including solid-phase peptide synthesis (SPPS) and bioconjugation. The Merg lab is invested in designing and synthesizing new classes of hybrid, multivalent peptide-based building blocks as a means to i) tap into unexplored peptide assembly spaces (e.g., fabrication of porous 2D and 3D architectures); ii) broaden the utility and capabilities of peptide-based materials; and iii) create biomaterials that can be tuned physically and chemically across the nano- to microscale.
To accomplish this, the Merg lab is developing new synthetic peptide macrocycles that are designed to self-assemble in multiple directions via programmable coiled coil-based interactions (Research Thrust 1). In addition, we are also investigating the construction of spherical, crystalline peptide particles (an unusual peptide assembly topology) that are assembled from amphiphilic collagen-mimetic peptides (aCMPs; Research Thrust 2). Research within the lab straddles the border of supramolecular chemistry, peptide chemistry, biochemistry, and materials chemistry.
TWO CURRENT RESEARCH THRUSTS:
- Research Thrust 1: Development of Multidimensional, Cyclic Peptide Tiles as Hierarchical Assembly Units
A major project goal within our lab is to expand the de novo protein/peptide assembly space through leveraging the full arsenal of peptide, protein, and bioconjugation chemistry to create fully synthetic protein-mimetic structures. In this thrust, we aim to establish a new class of cyclic peptide tiles that employ programmable and orthogonal coiled coil-based interactions to drive their controlled self-assembly into higher dimensional protein-based architectures.
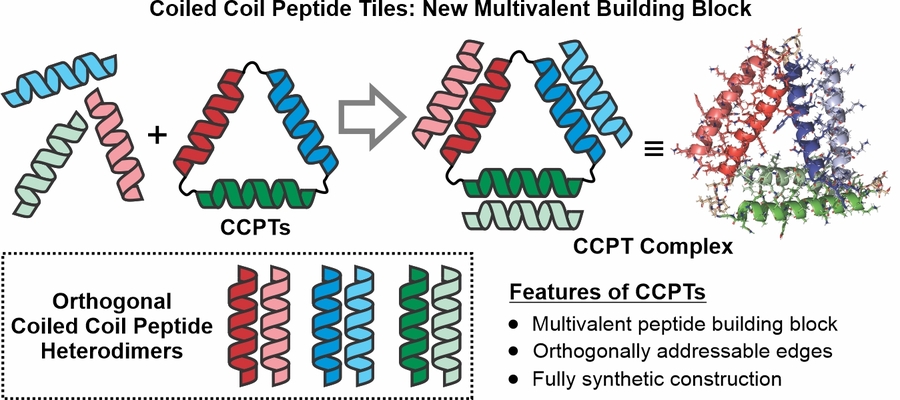
- Research Thrust 2: Fabrication of Crystalline Microparticles from Amphiphilic Collagen-Mimetic Peptides (aCMPs)
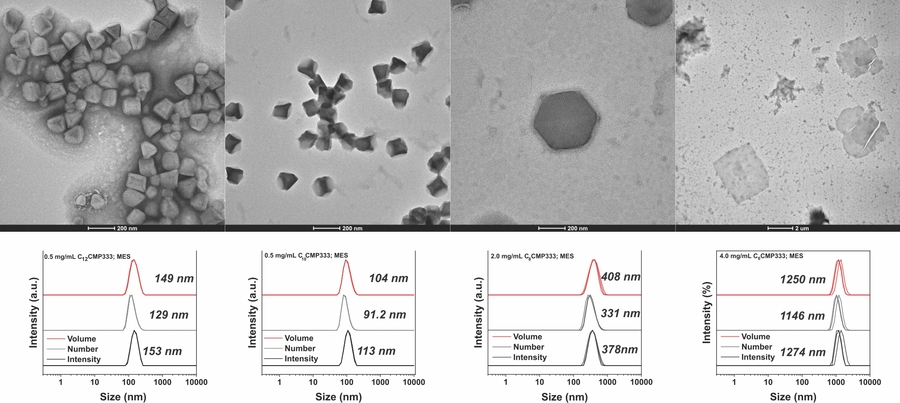
Our group is exploring the self-assembly of aCMPs into unique, close-packed crystalline structures with nano- to mesoscale physical dimensions. Currently CMP-based materials are primarily confined to nanosheets and fibers via the hierarchical organization of of collagen triple helices. The development of new topologies and architectures, such as the crystalline particles derived from aCMPs, will open up new applications for CMP-based nanostructures.
RESEARCH FUNDING:

