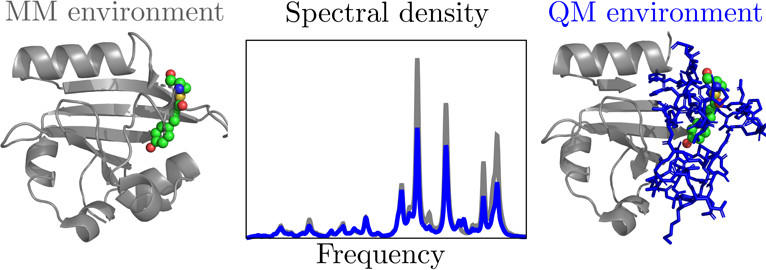
In this paper "The Influence of Electronic Polarization on the Spectral Density," the Isborn and Shi groups have come together to examine the role of polarization by the environment on the low and high frequency regions of the spectral density for a variety of condensed phase systems, including chromophores in different solvents and the photoactive yellow protein (PYP). We compare computed spectral densities and resulting linear absorption spectra and reorganization energies when using a standard fixed-point charge environment to using a fully polarizable large-scale quantum mechanical environment in the excited state calculations. We find that for some systems, e.g. Nile Red in weakly interacting solvents, the polarizable environment doesn’t strongly affect the spectral density and only provides a static shift of the spectrum, whereas for other systems, such as the PYP chromophore in water or protein, both the low-frequency and the high-frequency regions of the spectral density are affected, resulting in changes to both the static broadening and the vibronic coupling that determine spectral shape. We expect that this new physical insight will have broad appeal across chemistry, both to theorists who want to accurately model linear and nonlinear spectroscopy, as well as those studying excitation energy transfer pigment-protein complexes.
